With so many electronic devices in our daily lives, anyone who doesn’t have a good stockpile of common sized batteries is asking for trouble. While it is still possible to live without those electronic devices, we’ve become so accustomed to using them, and the convenience that they provide, that it would be difficult to get by without them, especially in the event of an emergency.
But there are more types of batteries today than ever before. So, between the increased types of batteries and the increased number of devices we all have, it can be challenging having all the batteries we need. If only we could make our batteries last longer; that would save us money, as well as allow us to use our survival devices longer in a crisis.
What if I were to tell you that you could? What if I were to say that “dead batteries” weren’t really dead?
Related: Will an EMP destroy batteries?
Are Dead Batteries Really Dead?
Let’s talk a little battery theory for a moment. Small batteries fall into two basic categories: rechargeable and single use. Obviously, you can recharge batteries that are designed for it and continue using them. That’s not what I’m talking about. What I’m talking about are the batteries designed for single use. We usually throw them away, once they are dead.
But what is dead?
For simplicity sake, let’s just talk about AA and AAA batteries. These are the most common sizes and whatever applies to them will also apply to all other single use batteries. These battery sizes, as well as C and D cells, are rated at a nominal 1.5 volts DC. I say “nominal” because new batteries actually have a higher charge, typically somewhere around 1.6 volts DC.
The devices we use generally have (use) a number of these batteries, as there is little that actually runs off of 1.5 volts (although there are some devices that do). To get more voltage, the batteries are connected in series. That means that the positive end of one battery (the one with the nipple on it) is connected to the negative end of the next (the flat end). Whenever we do that, the voltage of the batteries is added, increasing the voltage. So, we’ll put two batteries in to have a nominal 3 volts or 4 batteries to have a nominal 6 volts.

Batteries produce this electrical power by chemical reaction. As the device is used, it draws power from the batteries, gradually lowering the amount of available chemicals. As this level diminishes, the voltage that the battery produces drops as well.
So, that battery which started out somewhere around 1.6 volts, will keep dropping its voltage until it hits about 1.3 volts. At that point, the device usually stops working. This is the point at which we normally say that the battery is “dead.” But it really isn’t. The battery just has less power than it needs to have, in order to power our device. But perhaps it can be used for something else.
Getting More Out of the Batteries
Okay, so if those batteries still have some power in them, all we need is some way of getting it out. To do that, we’re going to do the exact same thing that we did inside the device; we’re going to connect the batteries in series. The only difference is, we’re going to connect more of them together, than we usually do. By doing this:

- 4 batteries provide us with 5.2 volts (1.3 x 4)
- 6 batteries provide us with 7.8 volts (1.3 x 6)
- 8 batteries provides us with 10.4 volts (1.3 x 8)
- 12 batteries provides us with 15.6 volts (1.3 x 12)
Obviously, these batteries can’t be put in the device this way, because there isn’t enough physical space for them inside the device. But many electronic devices have an external power connector, which we could connect to.
Many of today’s electronics are designed to be able to be recharged by a computer; connecting it to the USB connector. The USB connector on your computer provides 5 volts. So do the USB chargers that plug into the wall. The main difference is that the ones which plug into the wall will provide a lot more 5 volt power than you can pull out of a USB connector on your computer. That’s why some devices won’t charge when connected to a computer.
If you look at the battery in many of these devices (cell phones are a great example), it’s actually not a 5 volt battery, but a 3.6 to 3.8 volt battery. So, as long as we are providing more than 3.6 volts to the device, it will work. We can get more than that much power out of 4 dead AA batteries.
All we need, is a way of hooking all this up together; some sort of a battery pack that will allow us to connect the batteries together, along with a connector to attach to our electronic devices.
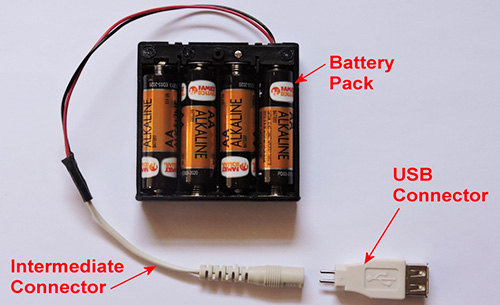
This battery pack holds 4 AA batteries. If we put in 4 dead AA batteries, that means we’ve got a total of 5.2 (1.3 x 4) volts available to us. There’s an intermediate connector that I’ve attached to it, in order to allow the same battery pack to provide power through a variety of connectors. Finally, since that’s enough to run those devices that charge off of a USB connector, I’ve added a USB connector.
This device will now work to charge or power a cell phone, tablet or digital camera; amongst a host of other devices. All I need is a USB to micro USB adapter, something that is quite common. So, I can continue using those batteries, until their output voltage drops to 0.867 volts each. Then the batteries will have to be replaced.
Related: Powering an Off the Grid House
But They Are Still Not Dead
Granted, 0.867 volts doesn’t sound like much, but it’s still a bit over half the battery’s nominal rating. So, we should be able to continue using them a while longer, just by using more batteries connected together in series, than what we had before.
If we connect six of these batteries together in a battery pack, we’ll be producing 5.2 volts. If we connect eight of them together, we’ll be producing 6.936. Either of these would work for powering those same USB devices. Since battery packs to hold six batteries are not that common, but battery packs for four and eight batteries are fairly common, we might be better off using the eight batteries.
Even though this sounds like too much power for the devices, it’s not. All electronic devices have a voltage regulator in them. They have to, as the amount of power the batteries provide isn’t constant. We’ve seen how it goes down. So, the “extra” voltage will be cut off, only allowing the amount of voltage that the device needs to pass through.
In real terms, there are limits to how much voltage we can give a device, before the voltage regulator can’t do its job. But that’s not a worry here, as we’re not going over that limit. Were we to hook 12 volts to a 5 volt device, however, it would probably be too much.
So, there you have it, a great way to save money on batteries, but more importantly, a great way to get more mileage out of your batteries in an emergency survival situation.
You may also like:
 EMP Myths and Facts. What’s Media Hype and What’s True?
EMP Myths and Facts. What’s Media Hype and What’s True?
This Hidden DIY Underground Bunker Will Save Your Life When SHTF (Video)
What to Keep in Your Car in Case of an EMP














This makes sense for heavy duty cells. A better solution for alkaline cells is a charger built for that chemistry. I have been using a Pure Energy charger for years. I get 6-10 reuses per disposable alkaline.
I use a 1.5 watt solar panel I paid 10 bucks for. I use alligator clips to a 4 AA wall plug charger . I get about 10 recharges to my batteries. I have used my regular charged alkaline batteries in my AM/FM radio for 6 hours a day and they are still producing output, with over a week of use. Recharged AA’s and AAA’s will hold a charge.
Can you provide more details? Do you just connect the clips to the plug prongs
lets see here, i have lots of AAA and AA powered devices, personal blenders, electric shavers , tools etc…
i have some usb power adapters that plug into 12 volt cigarette lighter recepticals and i have usb AAA and AA chargers that plug into these cigarette adapters. i use these to charge my alkaline batteries over and over again. i just do not drain them very low at first. yes, they hold less and less each time i recharge them, but im saving money. i have solar AAA/AA battery chargers and even recharge batteries by placing them near my lights at night, and im able to recoup some of my lighting expeience and i have other solar chargers too.
i also made a lead acid rejuvinator useing a big capacitor, a bridge rectifier, an 110 volt electric cord, a small plastic tool box, and some battery clamps. it plugs into household current and pulses 110+ volts dc at 60 pulses per second into batteries. by former boss had the plans to build some of these to salvage 6 volt golf car batteries at his golf car shop, to run the forklifts he uses for shop lifts. he could salvage the crap out of them with this devices. ive actualy reconditioned regular automobile batteries with this device to make a starting battery last 3 years after the battery tester said it was junk. i havent tried it yet but in cuba they charge hearing aid button cells with household current.
in prison we would use paper and tape to make paper tubes to series stack C or D batteries to power our radios and would charge them some what by placing them above the lights over the desk in our cells. that was 1970’s and 1980’s.
Do you have plans and parts list for these chargers?
I meant rejuvinators.
Living in da mountains, I find all of this essential. What a difference this has made.
What an eye-opener this article is! I have always felt a little guilty throwing away weak batteries, as if they were being wasted.
Please, write more about these clever energy-saving ideas and how-tos!